Wednesday, November 09, 2005
Sitio plasmidos
Para los que han subclonado y hecho sus plasmidos, esta herramienta es muy util y facil de usar para hacer mapas.
http://wishart.biology.ualberta.ca/PlasMapper/index.html
Saludos
J.
Tuesday, November 08, 2005
Clasificacion muerte celular
http://132.248.107.22:2062/cdd/journal/v12/n2s/full/4401724a.html
(requiere que pongan la clave de biomedicas)
Esta publicado en CDD, suplemento de noviembre, en el que, por cierto, vienen varias revisiones muy interesantes.
Friday, November 04, 2005
Buena noticia
Es aun version beta, pero ya tiene bastantes libros
http://print.google.com
pruebenlo!!
Monday, October 31, 2005
Nueva clasificacion
Ahora son 6 y bastante dificiles
The new classification recognizes 6 major clusters of organisms, rather than the 4 traditional Kingdoms. These clusters are 1) the Opisthokonta, grouping the animals, fungi, choanoflagellates, and Mesomycetozoa; 2) the Amoebozoa, grouping most traditional amoebae, slime moulds, many testate amoebae, some amoebo-flagellates, and several species without mitochondria; 3) the Excavata, grouping oxymonads, parabasalids, diplomonads, jakobids, and several other genera of heterotrophic flagellates, and possibly including the Euglenozoa and Heterolobosea; 4) the Rhizaria, grouping the Foraminifera, most of the traditional Radiolaria, and the Cercozoa with filose pseudopodia, such as many amoebo-flagellates and some testate amoebae; 5) the Archaeplastida, grouping the Glaucophyta, red algae, green algae, and Plantae; 6) the Chromalveolata, grouping the Alveolata (including ciliates, the dinoflagellates, and the Apicomplexa), cryptophytes, haptophytes, and stramenopiles (including brown algae, the diatoms, many zoosporic fungi, opalinids, amongst others)
Friday, October 21, 2005
Bid como mediador del daño al ADN
Sunday, October 16, 2005
Editorial de Nature
Vilma les ha enviado un editorial de Nature Medicine. El editor responsable es Juan Carlos Lopez, un mexicano emigrado a estados unidos. Creo que es importante que discutamos este punto en el blog, dado a que me parece que debemos estar conscientes de la imagen que tenemos al exterior y lo facil que es contribuir a ella.
Thursday, October 06, 2005
A propósito de ....
Nucleocytoplasmic transport in apoptosis
E. Ferrando-May.
Cell Death and Differentiation (2005) 12, 1263-1276.
Saturday, September 24, 2005
Peliculita RNAi
Tuesday, August 30, 2005
Bid en ciclo celular
A Role for Proapoptotic BID in the DNA-Damage Response
Sandra S. Zinkel,1,3,4,* Kristen E. Hurov,1,3 Christy Ong,1 Farvardean M. Abtahi,1 Atan Gross,2 and Stanley J. Korsmeyer1
Proapoptotic BID Is an ATM Effector in the DNA-Damage Response
Iris Kamer,1,4 Rachel Sarig,1,4 Yehudit Zaltsman,1 Hagit Niv,1 Galia Oberkovitz,1 Limor Regev,1 Gal Haimovich,1 Yaniv Lerenthal,2 Richard C. Marcellus,3 and Atan Gross1,*
Cell, Vol 122, 593-603, 26 August 2005
Thursday, August 25, 2005
The p53 pathway: positive and negative feedback loops
fidelity of DNA replication and cell division. A stress
signal is transmitted to the p53 protein by post-translational
modifications. This results in the activation of the
p53 protein as a transcription factor that initiates a
program of cell cycle arrest, cellular senescence or
apoptosis. The transcriptional network of p53-responsive
genes produces proteins that interact with a large number
of other signal transduction pathways in the cell and a
number of positive and negative autoregulatory feedback
loops act upon the p53 response. There are at least seven
negative and three positive feedback loops described here,
and of these, six act through the MDM-2 protein to
regulate p53 activity. The p53 circuit communicates with
the Wnt-beta-catenin, IGF-1-AKT, Rb-E2F, p38 MAP
kinase, cyclin-cdk, p14/19 ARF pathways and the cyclin
G-PP2A, and p73 gene products. There are at least three
different ubiquitin ligases that can regulate p53 in an
autoregulatory manner: MDM-2, Cop-1 and Pirh-2. The
meaning of this redundancy and the relative activity of
each of these feedback loops in different cell types or
stages of development remains to be elucidated. The
interconnections between signal transduction pathways
will play a central role in our understanding of cancer.
Oncogene (2005) 24, 2899–2908
Wednesday, August 24, 2005
Gran batalla en EU contra la teoría de la evolución de Darwin.
...Algo llamado "diseño inteligente" es la "teoría" avanzada como contrapropuesta a la teoría de Darwin, y después de una década de inversiones multimillonarias para financiar y promover académicos, publicaciones y esfuerzos de propaganda, las fuerzas antidarwinistas han logrado su objetivo: colocar su "teoría" al centro del debate nacional y punta de lanza de las llamadas "guerras culturales" de este país entre las fuerzas conservadoras fundamentalistas cristianas y todos los "otros", incluyendo casi a toda la comunidad científica establecida...
Les dejo el link.
Magali.
http://www.jornada.unam.mx
Monday, August 22, 2005
Discovery of the ubiquitin proteasome system and its involvement in apoptosis
Magali.
Revista
http://info.nature.com/cgi-bin24/DM/y/eUWy0JyZ8X0DK0GNW0Eq
Saturday, August 13, 2005
Malas noticias.
Inhibition of clonogenic tumor growth: a novel function of Smac contributing to its antitumor activity
Meike Vogler, Stavros Giagkousiklidis, Felicitas Genze, Juergen E Gschwend, Klaus-Michael Debatin and Simone Fulda
linkOncogene advance online publication, August 8, 2005; doi:10.1038/sj.onc.1208876
While second mitochondria derived activator of caspase (Smac) has been described to sensitize for apoptosis, its effect on cell viability in the absence of apoptotic stimuli has remained unclear. Here, we report that Smac inhibits clonogenic tumor growth by blocking random migration and proliferation and by enhancing apoptosis in a cell density and cell type dependent manner in SH-EP neuroblastoma cells. Inhibition of clonogenic survival by overexpression of full-length or processed Smac strictly depended on low cell density, and was reversible by replatement at high density. We discovered that Smac inhibits cell motility and random migration at low cell density. In addition, Smac enhanced apoptosis and inhibited protein, but not mRNA expression of XIAP, survivin and other short-lived proteins (FLIP, p21), indicating that Smac may globally inhibit protein expression. Also, Smac inhibited proliferation and increased polynucleation with no evidence for polyploidy, cell cycle arrest or senescence indicating that Smac impaired cell division. Interestingly, inhibition of clonogenic capacity by Smac occurred independent of its apoptosis promoting activity. By demonstrating that Smac restrains clonogenic tumor growth, our findings may have important implications for control of tumor growth and/or its metastatic spread. Thus, Smac agonists may be useful in cancer therapy, for example, for tumor control in minimal residual disease.
Ingrid tenia resultados compatibles con esto. Sin embargo, ellos fueron mas rapidos.
Monday, August 08, 2005
Importante para todos
| |
Nuclear factor-kappaB induced by doxorubicin is deficient in phosphorylation and acetylation and represses nuclear factor-kappaB-dependent transcription in cancer cells.
Cancer Res. 2005 May 15;65(10):4273-81.
The primary goal of chemotherapy is to cause cancer cell death. However, a side effect of many commonly used chemotherapeutic drugs is the activation of nuclear factor-kappaB (NF-kappaB), a potent inducer of antiapoptotic genes, which may blunt the therapeutic efficacy of these compounds. We have assessed the effect of doxorubicin, an anthracycline in widespread clinical use, on NF-kappaB activation and expression of antiapoptotic genes in breast cancer cells. We show that doxorubicin treatment activates NF-kappaB signaling and produces NF-kappaB complexes that are competent for NF-kappaB binding in vitro. Surprisingly, these NF-kappaB complexes suppress, rather than activate, constitutive- and cytokine-induced NF-kappaB-dependent transcription. We show that doxorubicin treatment produces RelA, which is deficient in phosphorylation and acetylation and which blocks NF-kappaB signaling in a histone deacetylase-independent manner, and we show that NF-kappaB activated by doxorubicin does not remain stably bound to kappaB elements in vivo. Together these data show that NF-kappaB signaling induced by doxorubicin reduces expression of NF-kappaB-dependent genes in cancer cells.
Articulos para el proximo lunes
Beth A.A. Weaver and Don W. Cleveland
Decoding the links between mitosis, cancer, and chemotherapy: The mitotic checkpoint, adaptation, and cell death
Cancer Cell 2005 8: 7-12
los articulos a revisar
Two distinct modes of cell death induced by doxorubicin: apoptosis and cell death through mitotic catastrophe accompanied by senescence-like phenotype. Young-Woo Eom, Mi Ae Kim, Seok Soon Park, Mi Jin Goo, Hyuk Jae Kwon, Seonghyang Sohn, Wook-Hwan Kim, Gyesoon Yoon and Kyeong Sook Choi Oncogene 24: 4765-4777;
Cdc2 and Cdk2 play critical roles in low dose doxorubicin-induced cell death through mitotic catastrophe but not in high dose doxorubicin-induced apoptosis.Biochem Biophys Res Commun. 2005 Sep 9;334(4):1014-21.
Friday, August 05, 2005
bcl-3 es reparador
Bcl3 reparador
en donde reportan el articulo de science sobre el papel de reparador de DNA por Bcl-3. Algo interesante seria checar las vias y las proteinas que interaccionan in silico para probarlas in vivo.
Thursday, August 04, 2005
Wednesday, August 03, 2005
Para los NFKBologos

El papel de la ubiquitacion y proteolisis en NFkB
Nature Cell Biology 7, 758 - 765 (2005)
Ubiquitin signalling in the NF-
 B pathway
B pathwayThe transcription factor NF-
 B controls many processes, including immunity, inflammation and apoptosis. Ubiquitination regulates at least three steps in the NF-
B controls many processes, including immunity, inflammation and apoptosis. Ubiquitination regulates at least three steps in the NF- B pathway: degradation of I
B pathway: degradation of I B (inhibitor of NF-
B (inhibitor of NF- B), processing of NF-
B), processing of NF- B precursors, and activation of the I
B precursors, and activation of the I B kinase (IKK). Recent studies have revealed several enzymes involved in the ubiquitination and deubiquitination of signalling proteins that mediate IKK activation through a degradation-independent mechanism.
B kinase (IKK). Recent studies have revealed several enzymes involved in the ubiquitination and deubiquitination of signalling proteins that mediate IKK activation through a degradation-independent mechanism.Ah! y felicidades a Juan Carlos por ser el primero en colocar un comentario.
Link Directo al articulo completo
Tuesday, August 02, 2005
Cambios
Cambie el formato, agregue unas fotos y puse los links a los articulos completos.
Saludos
J.
Saturday, July 30, 2005
TRAIL induce el corte de p65 por caspasa 3 para inducir apoptosis
Caspase-mediated p65 cleavage promotes TRAIL-induced apoptosis.
Cancer Res. 2005 Jul 15;65(14):6111-9
Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is cytotoxic to a wide variety of transformed cells, but not to most normal cells, implying potential therapeutic value against advanced cancer. However, signal transduction in TRAIL-mediated apoptosis is not clearly understood compared with other TNF family members. Specifically, it is not yet understood how TRAIL controls nuclear factor kappaB (NF-kappaB) activation and overcomes its anti-apoptotic effect. We explored the regulation of NF-kappaB activity by TRAIL and its role in apoptosis. TRAIL combined with IkappaBalpha-"superrepressor" induced potent apoptosis of SK-Hep1 hepatoma cells at low concentrations of TRAIL that do not independently induce apoptosis. Apoptosis by high concentrations of TRAIL was not affected by IkappaBalpha-superrepressor. Although TRAIL alone did not induce NF-kappaB activity, TRAIL combined with z-VAD significantly increased NF-kappaB activation. Analysis of the NF-kappaB activation pathway indicated that TRAIL unexpectedly induced cleavage of p65 at Asp97, which was blocked by z-VAD, accounting for all of these findings. p65 expression abrogated apoptosis and increased NF-kappaB activity in TRAIL-treated cells. Cleavage-resistant p65D97A further increased NF-kappaB activity in TRAIL-treated cells, whereas the COOH-terminal p65 fragment acted as a dominant-negative inhibitor. XIAP levels were increased by TRAIL in combination with z-VAD, whereas XIAP levels were decreased by TRAIL alone. Cleavage of p65 was also detected after FRO thyroid cancer cells were treated with TRAIL. These results suggest that TRAIL induces NF-kappaB activation, but simultaneously abrogates NF-kappaB activation by cleaving p65, and thereby inhibits the induction of anti-apoptotic proteins such as XIAP, which contributes to the strong apoptotic activity of TRAIL compared with other TNF family members.
Thursday, July 28, 2005
IKK1 vs IKK2?

IKK1 podria ser antagonista de la activacion de NFkB. Esto coincide con papers previos en los que los niveles relativos de IKK1 y 2 determinaban la activacion de NFkB y un articulo previo de IKK1 como inhibidor en macrofagos. En el caso del TIMP seria interesante analizar los niveles de ambas.
Zebrafish IκB Kinase 1 Negatively Regulates NF-κB Activity Ricardo G. Correa, Takaaki Matsui Vinay Tergaonkar, Concepcion Rodriguez-Esteban, Juan Carlos Izpisua-Belmonte and Inder M. Verma. Curr. Biol. 15: 26 July 2005, Pages 1291-1295
The IκB kinase (IKK) activity is critical for processing IκB inhibitory proteins and activating the NF-κB signaling, which is involved in a series of physiological and developmental steps in vertebrates [1, 2, 3 and 4]. The IKK activity resides in two catalytic subunits, IKK1 and IKK2, and two regulatory subunits, NEMO and ELKS [5, 6, 7 and 8]. IKK2 is the major cytokine-responsive IκB kinase [9, 10 and 11] because depletion of IKK1 does not interfere with the IKK activity [12, 13 and 14]. In fact, IKK1−/− mice display morphological abnormalities that are independent of its kinase activity and NF-κB activation [12, 13 and 14]. Hence, using zebrafish (Danio rerio) as a model, we examined the evolutionary role of IKK1 in modulating NF-κB. Ikk1−/− zebrafish embryos present head and tail malformations and, surprisingly, show upregulation of NF-κB-responsive genes and increased NF-κB-dependent apoptosis. Overexpression of ikk1 leads to midline structure defects that resemble NF-κB blockage in vivo [1]. Zebrafish Ikk1 forms complexes with NEMO that represses NF-κB in vertebrate cells. Indeed, truncation of its NEMO binding domain (NBD) restores NF-κB-dependent transcriptional activity and, consequently, the ikk1-overexpressing phenotype. Here, we report that Ikk1 negatively regulates NF-κB by sequestering NEMO from active IKK complexes, indicating that IKK1 can function as a repressor of NF-κB.
LINK A ARTICULO COMPLETO VIA BIBLIOTECA
Papilomavirus induce la expresion de IAP-2

Nuevo articulo publicado en oncogene describe la sobreexpresion de IAP-2 mediada por E6 y E7. Podria ser un marcador?
Human papillomavirus type 16 E6 and E7 oncoproteins upregulate c-IAP2 gene expression and confer resistance to apoptosis Huidong Yuan, Fenghua Fu, Jiaying Zhuo, Wei Wang, Junko Nishitani, Dong Sung An, Irvin S Y Chen and Xuan Liu Oncogene 24: 5069-5078; advance online publication, April 25, 2005
nhibition of apoptosis plays an important role in the cellular immortalization and transformation induced by E6 and E7 oncoproteins of human papillomavirus (HPV). Here, we report that the transcription of the inhibitor of apoptosis gene, cellular inhibitor of apoptosis protein 2, (c-IAP2), is significantly upregulated in HPV16 E6/E7-immortalized human oral keratinocytes (HOK16E6E7). Overexpression of E6/E7 from the high-risk HPV16 or 18, but not from the low-risk HPV6, activated c-IAP2 promoter. E6 from HPV16 and 18 played a major role in the activation. In addition, the induction of c-IAP2 transcription required nuclear factor- B activity. Overexpression of c-IAP2 in normal human oral keratinocyte conferred resistance to tumor necrosis factor-
B activity. Overexpression of c-IAP2 in normal human oral keratinocyte conferred resistance to tumor necrosis factor- (TNF-
(TNF- )/cycloheximide (CHX)-induced apoptosis, suggesting the increased c-IAP2 expression in HOK16E6E7 may protect the cells from TNF-
)/cycloheximide (CHX)-induced apoptosis, suggesting the increased c-IAP2 expression in HOK16E6E7 may protect the cells from TNF- -mediated cell death. Moreover, depletion of endogenous c-IAP2 using RNA interference in HOK16E6E7 induced apoptosis, indicating that c-IAP2 is necessary for HPV16 E6/E7-induced resistance to apoptosis and cell survival. Of note, high levels of c-IAP2 transcription were found in several HPV16- or HPV18-positive cancer cells, and depletion of c-IAP2 caused cell death in HPV18-positive HeLa cells. Thus, upregulation of c-IAP2 by E6 and E7 may confer resistance to apoptosis that is necessary for sustained growth of some HPV16- and HPV18-positive cancer cells.
-mediated cell death. Moreover, depletion of endogenous c-IAP2 using RNA interference in HOK16E6E7 induced apoptosis, indicating that c-IAP2 is necessary for HPV16 E6/E7-induced resistance to apoptosis and cell survival. Of note, high levels of c-IAP2 transcription were found in several HPV16- or HPV18-positive cancer cells, and depletion of c-IAP2 caused cell death in HPV18-positive HeLa cells. Thus, upregulation of c-IAP2 by E6 and E7 may confer resistance to apoptosis that is necessary for sustained growth of some HPV16- and HPV18-positive cancer cells.LINK AL ARTICULO COMPLETO VIA BIOMEDICAS
Wednesday, July 27, 2005
Elemento faltante

Este es un articulo importante. Las IAPs (en particular XIAP) son las inhibidoras naturales de las caspasas efectoras y la caspasa 9, de la via intrinseca. Sin embargo no existian las contrapartes de las caspasas de la via extrinseca. Algunos pensaban que la modulacion era mediada solo por FLIP, una proteina parecida a c. 8 sin actividad proteolitica. Sin embargo, El-Deiry identifico a las CARP 1 y 2 como las equivalentes a las IAPs en la via extrinseca. Las CARP habian sido identificadas antes como RHF1, un gen que estaba sobreexpresado en cancer de esofago, pero para el que no tenian funcion (esto no viene en el articulo). Ya tenemos primers de CARP1, que funcionan bien.
Suppression of caspase-8- and -10-associated RING proteins results in sensitization to death ligands and inhibition of tumor cell growth. Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):6170-5
The destruction of cellular targets during apoptosis is carried out by caspases, which are negatively regulated by the inhibitor of apoptosis proteins (IAP); however, death effector domain (DED) caspases of the extrinsic pathway are refractory to the IAP family. We have isolated a family of apoptotic inhibitors [caspases-8- and -10-associated RING proteins (CARPs)] that bind to and negatively regulate DED caspases. When overexpressed, CARPs, via an IAP-like RING domain, can contribute to the ubiquitin-mediated proteolysis of DED caspases. Furthermore, CARPs are rapidly cleaved during apoptosis. However, in tumors and tumor cell lines, they are overexpressed, and their silencing leads to restoration of efficient apoptosis via enhanced activation of DED caspases. Long-term inhibition of CARP expression results in suppression of cancer cell growth, highlighting their importance in tumor cell survival.
link al articulo completo
Tuesday, July 26, 2005
Revision de apoptosis intrinseca
Cell. 2005 Jun 3;121(5):671-4. Review.
For more than a decade, it has been apparent that apoptosis and other forms of cell death are often controlled at one or more crucial steps involving the mitochondria. Recent findings, including an elegant investigation in a recent issue of Cell (Hao et al., 2005
 ), have helped to elucidate fundamental aspects of this involvement while raising puzzling new questions about mitochondrial routes to cellular demise. The emerging, if preliminary, perspective these new studies provide may represent either a refinement of our views of how cells die or, perhaps, the beginnings of what amounts to a reformulation of our ideas.
), have helped to elucidate fundamental aspects of this involvement while raising puzzling new questions about mitochondrial routes to cellular demise. The emerging, if preliminary, perspective these new studies provide may represent either a refinement of our views of how cells die or, perhaps, the beginnings of what amounts to a reformulation of our ideas.Yo diria que la mejor revision de los ultimos dos años en este tema. Me gustaria que todos lo leyeran, sobre todo por nuestro resultados de smac y lo que planeo hacer a continuacion.
link a articulo via biomedicas
Revision de checkpoint mitotico
Beth A.A. Weaver1 and Don W. Cleveland
Cancer Cell, Vol 8, 7-12, July 2005
Disrupted passage through mitosis often leads to chromosome missegregation and the production of aneuploid progeny. Aneuploidy has long been recognized as a frequent characteristic of cancer cells and a possible cause of tumorigenesis. Drugs that target mitotic spindle assembly are frequently used to treat various types of human tumors. These lead to chronic mitotic arrest from sustained activation of the mitotic checkpoint. Here, we review the linkage between the mitotic checkpoint, aneuploidy, adaptation from mitotic arrest, and antimitotic drug-induced cell death.
Importante para revisar, sobre todo ruben, por lo de la crisis mitotica
LINK A ARTICULO COMPLETO VIA BIBLIOTECA BIOMEDICAS
Monday, July 25, 2005
Epithelial-mesenchymal transition (EMT)
"MMP-3-induced Rac1b stimulates formation of ROS, causing EMT and genomic instability," by Derek C. Radisky, Dinah D. Levy, Laurie E. Littlepage, Hong Liu, Celeste M. Nelson, Jimmie E. Fata, Devin Leake, Elizabeth L. Godden, Donna G. Albertson, M. Angela Nieto, Zena Werb, and Mina J. Bissell, appears in the 7 July 2005 issue of Nature.
Interesante por el hecho de que la modificacion externa, mediada por el estroma, puede incidir en la progresion del cancer. Cubre un vacio en el papel de la estromelisina en el cancer de mama.
LINK AL ARTICULO COMPLETO VIA BIOMEDICAS
Friday, July 22, 2005
Caspase-independent cell death
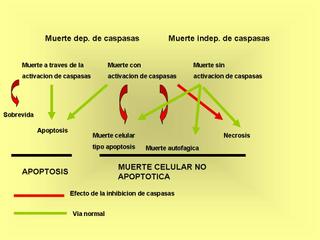
Una nueva revision publicada en Nature Medicine 11, 725 - 730 (2005) de G. Kroemer (el de AIF)
Caspase activation has been frequently viewed as synonymous with apoptotic cell death; however, caspases can also contribute to processes that do not culminate in cell demise. Moreover, inhibition of caspases can have cytoprotective effects. In a number of different models, caspase inhibition does not maintain cellular viability and instead shifts the morphology of death from apoptosis to nonapoptotic pathways. Here, we explore the contribution of caspases to cell death, either as upstream signals or as downstream effectors contributing to apoptotic morphology, as well as alternative strategies for cell death inhibition. Such alternative strategies may either target catabolic hydrolases or be aimed at preventing mitochondrial membrane permeabilization and its upstream triggers
LINK AL ARTICULO COMPLETO VIA BIOMEDICAS